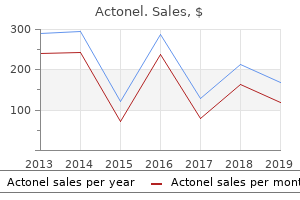
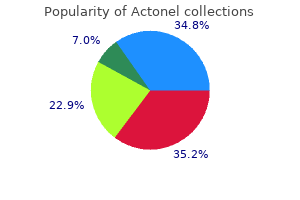
"Buy actonel 35 mg lowest price, medications rheumatoid arthritis".
By: R. Kayor, M.B.A., M.B.B.S., M.H.S.
Co-Director, Rutgers Robert Wood Johnson Medical School
Sequence numbers should be reassigned in the database if the facility learns later of an unaccessioned tumor that would affect the sequence symptoms 2016 flu buy generic actonel on line. Code the sequence number of the colon cancer to medications before surgery discount actonel 35 mg fast delivery 02 and change the sequence number of the lung cancer to medicine hat jobs discount actonel 35mg line 01. A person was diagnosed with breast cancer in April 2010 and metastasis to the lungs in June 2018. Since the lung is a metastatic site and not a second primary, it would not be abstracted. In 2019, this person developed a benign meningioma in the temporal area of the brain. The sequence number of the left forearm would not be sequenced, abstracted or reported. Do not include metastatic lesions or the primary currently being reported in this field. The patient had a history of duct cell carcinoma of the left breast in 2005 and is admitted in 2018 for adenocarcinoma of the lung. Complete an abstract on the lung tumor, and document duct cell carcinoma of left breast in 2005 in this field. The patient has a history of prostate cancer, no date or specific morphology is given. This field may be left blank if the sequence number is 00 for a malignant neoplasm or 60 for a non-malignant neoplasm. Explanation this item is used in financial analysis and as an indicator for quality and outcome analyses. Code the first insurance mentioned when multiple insurance carriers are listed in one admission record. Code the type of the insurance reported closest to the date of diagnosis when there are multiple insurance carriers reported from multiple admissions and/or multiple physician encounters. Note: Codes 21 and 65-68 are to be used for patients diagnosed on or after January 1, 2006. State government administered insurance for persons who are uninsured, below the poverty level, or covered under entitlement programs. Federal government funded insurance for persons who are 65 years of age or older, or are chronically disabled (Social Security insurance eligible). Federal government Medicare insurance with State Medicaid administered supplement. Department of Defense program providing supplementary civilian-sector hospital and medical services beyond a military treatment facility to military dependents, retirees, and their dependents. A patient has managed Medicare listed in Insurance #1 and Medicaid listed as Insurance #2. Code the Primary Payer at Diagnosis as 99 because the information from the facility where originally diagnosed is not available. If no height was listed on the date of diagnosis, use the height recorded on the date closest to the date of diagnosis and before treatment was started. If the information is not available use code 99 (Unknown) Note: An online conversion calculator is available at manuelsweb. If no weight was listed on the date of diagnosis, please use the weight recorded on the date closest to the date of diagnosis and before treatment was started. If the medical record only indicates "No," use code 9 (Unknown/not stated/no smoking specifics provided) rather than code 0 (Never used). Explanation the date of initial diagnosis is essential in the analysis of staging and treatment of the cancer, for epidemiology purposes, and for outcomes analysis. The timing for staging and treatment of cancer begins with the date of initial diagnosis for cancer. Example: A mammogram done in January 2018 shows that the patient has a malignancy in the upper outer quadrant of the right breast, but the day is unknown. The initial diagnosis date may be from a clinical diagnosis, for example, when a radiologist views a chest x-ray and the diagnosis is lung carcinoma. If later confirmed by a pathology specimen, the diagnosis date remains the date of the initial clinical diagnosis. However, if the radiologist states suspicious for malignancy (not neoplasm) in his/her impression, the case is reportable and the date of the mammogram would be considered the date of initial diagnosis for breast cancer. The date of diagnosis based on a pathology report should be the date the specimen was taken, not the date the pathology report was read or created.
Diseases
- Hydrocephalus costovertebral dysplasia Sprengel anomaly
- Motor neuro-ophthalmic disorders
- Rhabdomyosarcoma 2
- Gelineau disease
- Paraomphalocele
- Blepharo naso facial syndrome Van maldergem type
- Oligomeganephrony
- Contact dermatitis, irritant
- Osteonecrosis
- Thanos Stewart Zonana syndrome
Perform slit-lamp and ophthalmoscopic examination of patients with an ocular tumor treatment 3rd stage breast cancer buy actonel 35 mg free shipping. Describe diagnostic techniques for ocular tumors (eg medications zofran buy actonel discount, examination under anesthesia for pediatric tumors symptoms of ebola buy actonel online now, imaging, biopsy, laboratory tests, oncology referral). Describe indications (eg, biopsy for lymphoma) and contraindications (eg, biopsy for retinoblastoma) for the various diagnostic techniques. Describe the management options for ocular tumors with indications and contraindications for each form of management. Perform slit-lamp examination, gonioscopy, and indirect ophthalmoscopy to diagnose and localize ocular tumors. Perform enucleation, obtaining long optic nerve if appropriate, or refer to a subspecialist for this surgery if necessary. Collaborate with subspecialist in the preoperative care and referral of selected patients with an ocular tumor, avoiding potential pitfalls. Provide short-term and long-term postoperative care to patients with an ocular tumor, collaborating with a subspecialist and other health care workers as appropriate. Investigate and manage ocular complications as appropriate (eg, radiation retinopathy, macular edema, cataract, glaucoma). Interpret the results of laboratory investigations and adjust management accordingly. Discuss prognosis and various management options with patients and their families in a detailed, ethical, and compassionate manner. Describe the applied surgical anatomy, histology, and physiology of the eye and ocular adnexa with relevance to ocular oncology. Paraneoplastic disease (eg, Bilateral diffuse uveal melanocytic proliferation)** g. Describe relevant pathological techniques (eg, fixation, histology, immunohistochemistry). List the differential diagnosis of each tumor, and describe the investigational approach for each condition. Describe how statistics can be applied to ocular oncology (eg, survival analysis). Describe the methods, risks, and benefits of tumor biopsy and how these can be avoided (eg, biopsy of retinoblastoma, incisional biopsy of conjunctival tumor). Perform or request appropriate examinations and investigations according to differential diagnosis. Perform or refer for treatment for conjunctival or intraocular tumors, demonstrating awareness of the indications, contraindications, and complications of each treatment and having skill to administer short-term and long-term postoperative care:** a. Interpret results of relevant laboratory tests and communicate results to patients, relatives, and health care workers; and adjust patient management accordingly. Communicate prognosis with patients, relatives, and health care workers; and adjust patient management accordingly in collaboration, if necessary, with a subspecialist. Assist patients with selecting the most appropriate management in collaboration, if necessary, with a subspecialist in ocular oncology. Provide or organize appropriate psychological support, demonstrating empathy and an adequate awareness of the principles of this aspect of care (eg, giving bad news). Collaborate with subspecialists and other health care professionals to provide patientfocused care. Develop protocols and infrastructure for practice-based learning and improvement (eg, access to information, outcomes data). Very Advanced Level Goals: Subspecialist Subspecialist equivalent: senior ophthalmologist responsible for ocular oncology, either parttime or full-time, who receives ocular oncology patient referrals. Describe the applied surgical anatomy, histology, and embryology of the eye and ocular adnexa with relevance to ocular oncology. Describe the applied physiology of the eye and adnexa with relevance to ocular oncology. Describe the relevant staging and grading systems for ocular tumors (with ability to use appropriate methods as necessary, using appropriate references sources): a.
Syndromes
- Late-onset Krabbe disease begins in late childhood or early adolescence.
- Promethazine (to treat nausea and vomiting)
- Dream-like hallucinations between sleep and wakefulness. They involve seeing or hearing, and possibly other senses.
- A strip of bone is usually removed where two sutures connect. This is called a strip craniectomy. Sometimes, larger pieces of bone must also be removed. This is called synostectomy. Parts of these bones may be changed or reshaped when they are removed. Then, they are put back. Other times, they are not.
- Neck or brain (stroke)
- Speech changes
- Chloral hydrate
- Your child has abscess or growth on their tonsils.
- Inflammatory bowel disease
- Decreased testosterone levels in men
At 440 mg/m2/d one pt experienced drug-related Gr3 dehydration and mucosal inflammation symptoms 4 days post ovulation generic 35mg actonel with mastercard, and Gr4 febrile neutropenia; a second pt experienced Gr4 febrile neutropenia treatment 34690 diagnosis purchase cheap actonel on-line. Longitudinal plasma samples symptoms 7 weeks pregnancy discount actonel 35 mg otc, collected from 52 pts, were subjected to sequencing using a panel consisting of 168 lung cancer-related genes. Results: Genomic profile prior to the initiation of osimertinib revealed that mutations participating in cell cycle (14 pts, p = 0. With a median follow-up of 168 d (ranged from 40 - 550 d), 32 pts experienced radiological disease progression. Among them, 11 (34%) experienced molecular progression reflected by emergency of new mutation or increased allelic frequency of existing mutation prior to radiological progression, with an average leading time of 74 days. Methods: Progastrin levels were measured in the blood samples of 1319 patients with 12 different cancer origins, and compared to those of 557 asymptomatic 18-75 years old blood donors. Results: Compared to healthy blood donors, progastrin was found at higher concentrations in the plasma of cancer patients: median 4. The longitudinal progastrin changes during treatments, suggest relationships to tumor burden, and potential monitoring value. It may change the future paradigms about screening (in particular for populations at higher or lower risks of cancer), cancer diagnostic & monitoring. The most observed metastatic sites were bone (37%), lymph nodes (29%), lung (27%) and liver (25%). Methods: We prospectively collected clinical data and blood from 69 advanced cancer patients (28 lung, 25 breast, 16 other). Blood was collected at baseline prior to initiation of a new treatment and at one or two additional timepoints (median 21 and 42 days). Patients were treated with the following therapies: chemotherapy (37), immunotherapy (17), endocrine (9), or targeted therapy (6). Positive predictive value was 100% for disease progression and negative predictive value was 78%. These findings were consistent in subset analyses of patients with lung (logrank P = 2x10-5), breast (log-rank P = 3x10-4), and those treated with immunotherapy (log-rank P = 5x10-6). These findings were consistent across multiple tumor types and treatments, including immunotherapy patients. Once validated, this dynamic, predictive, blood-based biomarker could aid in clinical decision making for early treatment change as a novel and cost-effective approach. Methods: We prospectively collected clinical data and blood from 28 patients with metastatic malignancies (13 lung, 11 breast, 4 other). Blood was drawn prior to start of a new treatment, after first cycle (median 30 days), and/or second cycle (median 57 days). By tracking how methylation levels deviate from unaffected individuals, from baseline to subsequent timepoints, we classified patients as either progressors (greater deviance) or non-progressors. Main treatment regimens were chemo- (N = 12), immuno- (6), endocrine (5), or targeted-therapy (5). For the remaining patients, 18 of 20 did not progress (90% negative predictive value). Thus, sensitivity for the assay for identifying progression was 78% and specificity was 95%. However ~20% of patients are intrinsically resistant and all patients eventually acquire resistance to these therapies. First Author: Yumi Kim, Department of Surgery, Seoul National University College of Medicine, Seoul, South Korea Background: Breast cancer is the most frequently diagnosed cancer and the most leading cause of cancer-related deaths among women worldwide. Although screening mammography is available, there is an ongoing interest in improved early detection and prognosis. Research investigating biomarkers for early detection, prognosis and the prediction of treatment responses in breast cancer is rapidly expanding. However, no validated biomarker currently exists for use in routine clinical practice, and breast cancer detection and management remains dependent on invasive procedures We aimed to develop biomarker for diagnosis of breast cancer in the real clinical practice by using proteomics technology. Methods: Based on our previous studies, we performed verification and validation of 124 candidate proteins by using proteomics approach. Among these 124 candidate proteins, the three proteins (neural cell adhesion molecule L1-like protein, apolipoprotein C-1, carbonic anhydrase-1) with highest statistical significance were selected.